
CFDA Listing
CFDA Listing Procedure(MD class I, Imported)
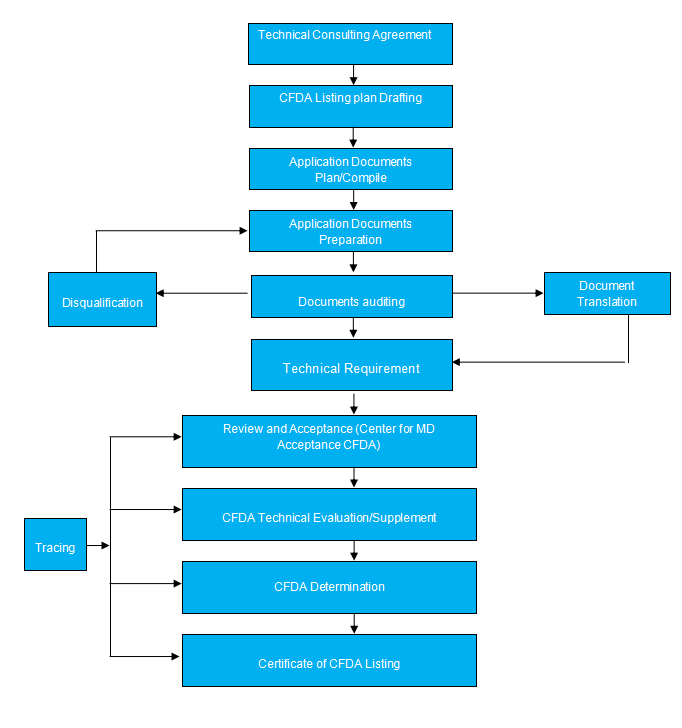
CFDA Requirement
1. Application for MD registration
2. Risk Analysis Report
3. Product Technical Request
4. Testing Report
5. Clinical evaluation
6. Information of the Manufacturer
7. Users instruction and samples of least packing and label
8. Proof of Origin: Marketing approval /Authorization of Agency/ Notarization ;
9. Declaration of conformity
Jiushun Technical support
1. Provide guidance on registration process and technology according to CFDA regulations
2. Provide documental samples according to CFDA regulations
3. Compile Product Technical Request
4. Assist with testing
5. Provide guidance on Proof of Origin
6. Examine and verify documents and guide to adjust/submit documents to CFDA and follow up
7. Paid translation service
Why Jiushun Management?
Jiushun Management, focused on Medical Device Registration, Certification for 20 years, provided high quality service for more than 5,000 global customers.
The MD industry focus degrees, regulatory familiarity, and rich experience, determine our high efficiency and professionalism.
Our professional technical support, from the early regulations, processes guidance, documentation preparation, to testing assistance, review tracking, as well as years of MD industry resource integration, will greatly shorten the period of your product approval, and well practice our principle "Jiushun Management, create value for you. ".





